Main Difference
The main difference between Vaporization and Evaporation is that the Vaporization is a phase transition from the liquid phase to vapor (either through evaporation or boiling) and Evaporation is a type of vaporization of a liquid that occurs from its surface; surface phenomenon
-
Vaporization
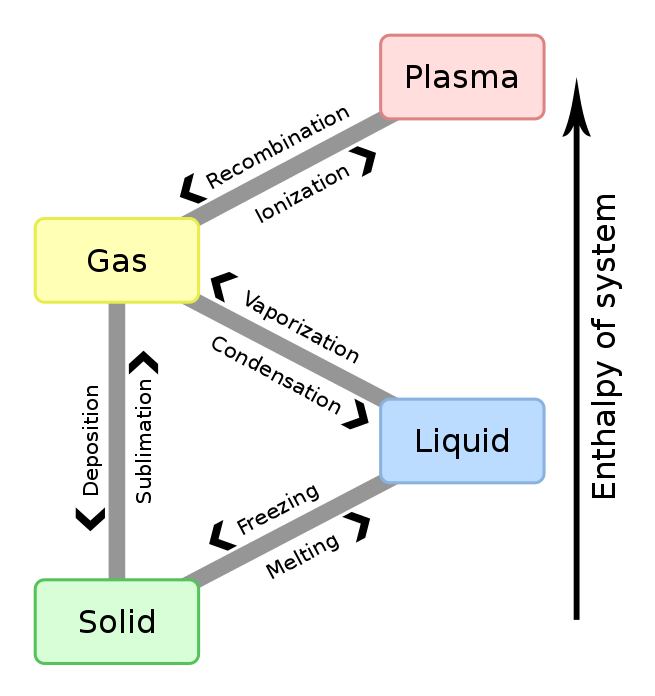
Vaporization (or vaporisation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation and boiling. Evaporation is a surface phenomenon, whereas boiling is a bulk phenomenon.
Evaporation is a phase transition from the liquid phase to vapor (a state of substance below critical temperature) that occurs at temperatures below the boiling temperature at a given pressure. Evaporation occurs on the surface. Evaporation only occurs when the partial pressure of vapor of a substance is less than the equilibrium vapor pressure. For example, due to constantly decreasing or negative pressures, vapor pumped out of a solution will leave behind a cryogenic liquid.
Boiling is also a phase transition from the liquid phase to gas phase, but boiling is the formation of vapor as bubbles of vapor below the surface of the liquid. Boiling occurs when the equilibrium vapor pressure of the substance is greater than or equal to the environmental pressure. The temperature at which boiling occurs is the boiling temperature, or boiling point. The boiling point varies with the pressure of the environment.
Sublimation is a direct phase transition from the solid phase to the gas phase, skipping the intermediate liquid phase. Because it does not involve the liquid phase, it is not a form of vaporization.
The term vaporization has also been used in a colloquial or hyperbolic way to refer to the physical destruction of an object that is exposed to intense heat or explosive force, where the object is actually blasted into small pieces rather than literally converted to gaseous form. Examples of this usage include the “vaporization” of the uninhabited Marshall Island of Elugelab in the 1952 Ivy Mike thermonuclear test.At the moment of a large enough meteor or comet impact, bolide detonation, a nuclear fission, thermonuclear fusion, or theoretical antimatter weapon detonation, a flux of so many gamma ray, x-ray, ultraviolet, visual light and heat photons strikes matter in a such brief amount of time (a great number of high-energy photons, many overlapping in the same physical space) that all molecules lose their atomic bonds and “fly apart”. All atoms lose their electron shells and become positively charged ions, in turn emitting photons of a slightly lower energy than they had absorbed. All such matter becomes a gas of nuclei and electrons which rise into the air due to the extremely high temperature or bond to each other as they cool. The matter vaporized this way is immediately a plasma in a state of maximum entropy and this state steadily reduces via the factor of passing time due to natural processes in the biosphere and the effects of physics at normal temperatures and pressures.
A similar process occurs during ultrashort pulse Laser ablation, where the high flux of incoming electromagnetic radiation strips the target material’s surface of electrons, leaving positively charged atoms which undergo a coulomb explosion.
-
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. The surrounding gas must not be saturated with the evaporating substance. When the molecules of the liquid collide, they transfer energy to each other based on how they collide with each other. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling.On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is saturated.
Evaporation is an essential part of the water cycle. The sun (solar energy) drives evaporation of water from oceans, lakes, moisture in the soil, and other sources of water. In hydrology, evaporation and transpiration (which involves evaporation within plant stomata) are collectively termed evapotranspiration. Evaporation of water occurs when the surface of the liquid is exposed, allowing molecules to escape and form water vapor; this vapor can then rise up and form clouds. With sufficient energy, the liquid will turn into vapor.
-
Vaporization (noun)
A conversion of a solid or a liquid into a gas.
-
Vaporization (noun)
A destruction of something by turning it into vapor.
-
Evaporation (noun)
The process of a liquid converting to the gaseous state.
-
Evaporation (noun)
The process in which all or a portion of liquid (in a container) is turned into vapour, in order to increase the concentration of solid matter in the mixture.
-
Evaporation (noun)
That which is evaporated; vapor.
-
Evaporation (noun)
the process of turning from liquid into vapour
“snow cover prevents evaporation of water from the soil”
-
Evaporation (noun)
the process of something abstract ceasing to exist
“the police’s attempt to dictate public policy led to a sudden evaporation of support”
“thousands of employees witnessed the rapid evaporation of their retirement plans”