Main Difference
The main difference between Nylon and Polyamide is that the Nylon is a family of synthetic polymers originally developed as textile fibers and Polyamide is a macromolecule with repeating units linked by amide bonds.
-
Nylon
Nylon is a generic designation for a family of synthetic polymers, based on aliphatic or semi-aromatic polyamides.
Nylon is a thermoplastic silky material
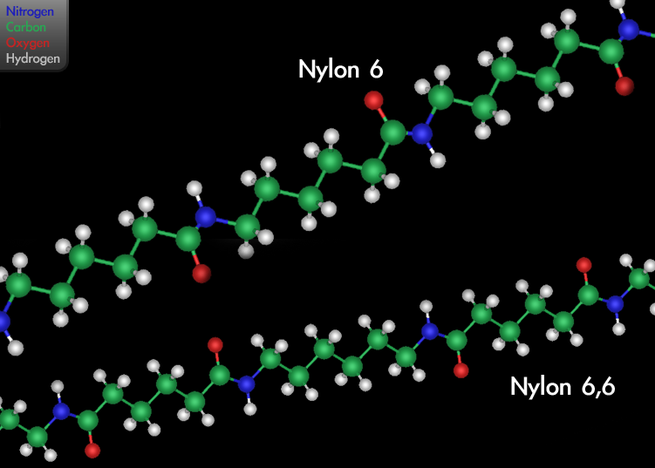
that can be melt-processed into fibers, films or shapes.Nylon was the first commercially successful synthetic thermoplastic polymer. DuPont began its research project in 1930.
The first example of nylon (nylon 6,6) was produced using diamines on February 28, 1935, by Wallace Hume Carothers at DuPont’s research facility at the DuPont Experimental Station. In response to Carothers’ work, Paul Schlack at IG Farben developed nylon 6, a different molecule based on caprolactam, on January 29, 1938.Nylon was first used commercially in a nylon-bristled toothbrush in 1938, followed more famously in women’s stockings or “nylons” which were shown at the 1939 New York World’s Fair and first sold commercially in 1940. During World War II, almost all nylon production was diverted to the military for use in parachutes and parachute cord. Wartime uses of nylon and other plastics greatly increased the market for the new materials.Nylon is made of repeating units linked by amide links similar to the peptide bonds in proteins.
Commercially, nylon polymer is made by reacting monomers which are either lactams, acid/amines or stoichiometric mixtures of diamines (-NH2) and diacids (-COOH). Mixtures of these can be polymerized together to make copolymers. Nylon polymers can be mixed with a wide variety of additives to achieve many different property variations.
Nylon polymers have found significant commercial applications in fabric and fibers (apparel, flooring and rubber reinforcement), in shapes (molded parts for cars, electrical equipment, etc.), and in films (mostly for food packaging).
-
Polyamide
A polyamide is a macromolecule with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made through step-growth polymerization or solid-phase synthesis yielding materials such as nylons, aramids, and sodium poly(aspartate). Synthetic polyamides are commonly used in textiles, automotive applications, carpets and sportswear due to their high durability and strength. The transportation manufacturing industry is the major consumer, accounting for 35% of polyamide (PA) consumption.
-
Nylon (noun)
Originally, the DuPont company trade name for polyamide, a copolymer whose molecules consist of alternating diamine and dicarboxylic acid monomers bonded together; now generically used for this type of polymer.
-
Nylon (noun)
A stocking originally fabricated from nylon; also used generically for any long, sheer stocking worn on a woman’s legs.
“They left the strip club when they discovered the ladies only stripped down to their nylons.”
-
Polyamide (noun)
Any of a range of polymers containing amide (or peptide) repeat units; examples include proteins and nylon.
-
Polyamide (noun)
a synthetic polymer of a type made by the linkage of an amino group of one molecule and a carboxylic acid group of another, including many synthetic fibres such as nylon.