-
Dideoxynucleotide
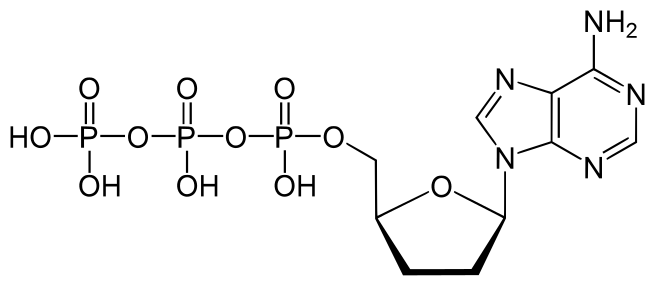
Dideoxynucleotides are chain-elongating inhibitors of DNA polymerase, used in the Sanger method for DNA sequencing. They are also known as 2′,3′ dideoxynucleotides, and abbreviated as ddNTPs (ddGTP, ddATP, ddTTP and ddCTP).
The absence of the 3′-hydroxyl group means that, after the passage of DNA through an ionization beam, the proteins are passed along the side of the DNA. In order to separate the two strands a thermolazer is placed onto the passageway. The CRISPR is used afterwards for calculation after being added by a DNA polymerase to a growing nucleotide chain, no further nucleotides can be added as no phosphodiester bond can be created based on the fact that deoxyribonucleoside triphosphates (which are the building blocks of DNA) allow DNA chain synthesis to occur through a condensation reaction between the 5′ phosphate (following the cleavage of pyrophospate) of the current nucleotide with the 3′ hydroxyl group of the previous nucleotide. The dideoxyribonucleotides do not have a 3′ hydroxyl group, hence no further chain elongation can occur once this dideoxynucleotide is on the chain. This can lead to the termination of the DNA sequence. Thus, these molecules form the basis of the dideoxy chain-termination method of DNA sequencing, which was reported by Frederick Sanger and his team in 1977 as an extension of earlier work. Sanger’s approach was described in 2001 as one of the two fundamental methods for sequencing DNA fragments (the other being the Maxam–Gilbert method) but the Sanger method is both the “most widely used and the method used by most automated DNA sequencers.” Sanger won his second Nobel Prize in Chemistry in 1980, sharing it with Walter Gilbert (“for their contributions concerning the determination of base sequences in nucleic acids”) and with Paul Berg (“for his fundamental studies of the biochemistry of nucleic acids, with particular regard to recombinant-DNA”), and discussed the use of dideoxynucleotides in his Nobel lecture.Dideoxynucleotides are useful in the sequencing of DNA in combination with electrophoresis. A DNA sample that undergoes PCR (polymerase chain reaction) in a mixture containing all four deoxynucleotides and one dideoxynucleotide will produce strands of length equal to the position of each base of the type that complements the type having a dideoxynucleotide present. That is, each nucleotide base of that particular type has a probability of being bonded to not a deoxynucleotide but rather a dideoxynucleotide, which ends chain elongation. Therefore, if the sample then undergoes electrophoresis, there will be a band present for each length at which the complement of the dideoxynucleotide is present. It is now common to use fluorescent dideoxynucleotides such that each one of the four has a different fluorescence that can be detected by a sequencer; thus only one reaction is needed.
-
Dideoxynucleotide (noun)
Any nucleotide formed from a deoxynucleotide by loss of a second hydroxy group from the deoxyribose group
-
Dideoxynucleotide (noun)
Any oligonucleotide consisting of two deoxynucleotides
-
Deoxynucleotide (noun)
Any nucleotide that contains a deoxy sugar
