Main Difference
The main difference between Buoyancy and Density is that the Buoyancy is a upward force that opposes the weight of an object immersed in fluid and Density is a mass per unit volume.
-
Buoyancy
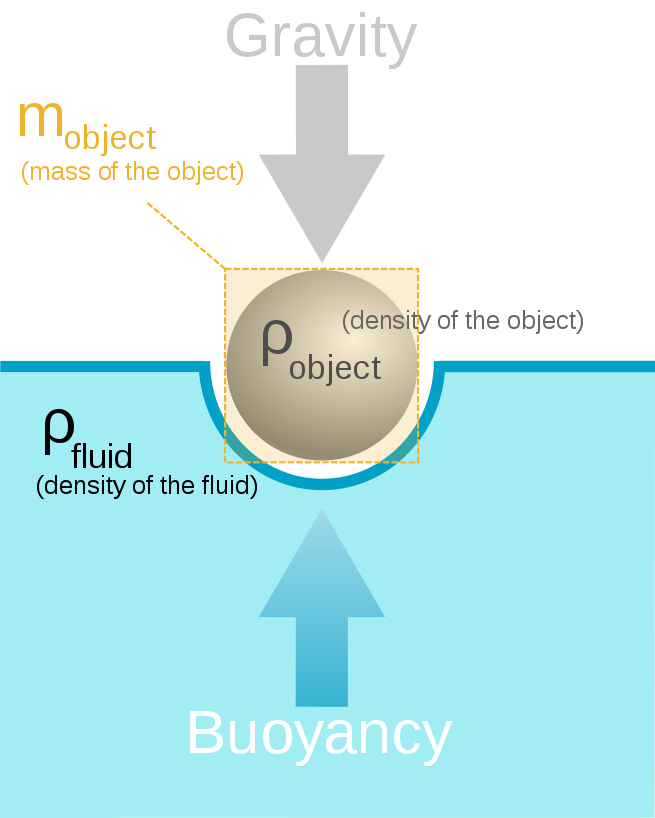
Buoyancy () or upthrust, is an upward force exerted by a fluid that opposes the weight of an immersed object. In a column of fluid, pressure increases with depth as a result of the weight of the overlying fluid. Thus the pressure at the bottom of a column of fluid is greater than at the top of the column. Similarly, the pressure at the bottom of an object submerged in a fluid is greater than at the top of the object. The pressure difference results in a net upward force on the object. The magnitude of the force is proportional to the pressure difference, and (as explained by Archimedes’ principle) is equivalent to the weight of the fluid that would otherwise occupy the volume of the object, i.e. the displaced fluid.
For this reason, an object whose average density is greater than that of the fluid in which it is submerged tends to sink. If the object is less dense than the liquid, the force can keep the object afloat. This can occur only in a non-inertial reference frame, which either has a gravitational field or is accelerating due to a force other than gravity defining a “downward” direction.The center of buoyancy of an object is the centroid of the displaced volume of fluid.
-
Density
The density, or more precisely, the volumetric mass density, of a substance is its mass per unit volume. The symbol most often used for density is ρ (the lower case Greek letter rho), although the Latin letter D can also be used. Mathematically, density is defined as mass divided by volume:
ρ
=
m
V
{displaystyle rho ={frac {m}{V}}}
where ρ is the density, m is the mass, and V is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight.
For a pure substance the density has the same numerical value as its mass concentration.
Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure but certain chemical compounds may be denser.
To simplify comparisons of density across different systems of units, it is sometimes replaced by the dimensionless quantity “relative density” or “specific gravity”, i.e. the ratio of the density of the material to that of a standard material, usually water. Thus a relative density less than one means that the substance floats in water.
The density of a material varies with temperature and pressure. This variation is typically small for solids and liquids but much greater for gases. Increasing the pressure on an object decreases the volume of the object and thus increases its density. Increasing the temperature of a substance (with a few exceptions) decreases its density by increasing its volume. In most materials, heating the bottom of a fluid results in convection of the heat from the bottom to the top, due to the decrease in the density of the heated fluid. This causes it to rise relative to more dense unheated material.
The reciprocal of the density of a substance is occasionally called its specific volume, a term sometimes used in thermodynamics. Density is an intensive property in that increasing the amount of a substance does not increase its density; rather it increases its mass.
-
Buoyancy (noun)
The upward force on a body immersed or partly immersed in a fluid.
-
Buoyancy (noun)
The ability of an object to stay afloat in a fluid.
-
Buoyancy (noun)
Resilience or cheerfulness.
-
Density (noun)
A measure of the mass of matter contained by a unit volume.
-
Density (noun)
The ratio of one quantity to another quantity.
“The number of particles per unit volume of a specified volume can be considered to be the particle density for the specified volume.”
-
Density (noun)
The probability that an event will occur, as a function of some observed variable.