
Main Difference
The main difference between Antigen and Antibody is that the Antigen is a molecule capable of inducing an immune response (to produce an antibody) in the host organism and Antibody is a large Y-shaped protein produced by B-cells, used by the immune system; large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses.
-
Antigen
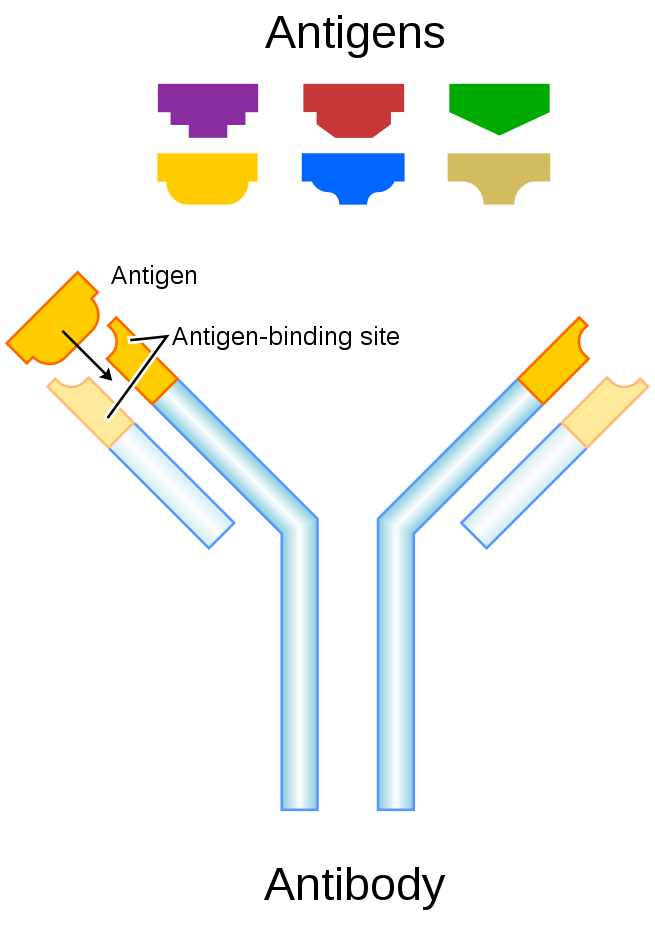
In immunology, antigens (Ag) are structures (aka substances) specifically bound by antibodies (Ab) or a cell surface version of Ab ~ B cell antigen receptor (BCR). The term antigen originally described a structural molecule that binds specifically to an antibody only in the form of native antigen. It was expanded later to refer to any molecule or a linear molecular fragment after processing the native antigen that can be recognized by T-cell receptor (TCR). BCR and TCR are both highly variable antigen receptors diversified by somatic V(D)J recombination. Both T cells and B cells are cellular components of adaptive immunity. The Ag abbreviation stands for an antibody generator.Antigens are “targeted” by antibodies. Each antibody is specifically produced by the immune system to match an antigen after cells in the immune system come into contact with it; this allows a precise identification or matching of the antigen and the initiation of a tailored response. The antibody is said to “match” the antigen in the sense that it can bind to it due to an adaptation in a region of the antibody; because of this, many different antibodies are produced, each able to bind a different antigen while sharing the same basic structure. In most cases, an adapted antibody can only react to and bind one specific antigen; in some instances, however, antibodies may cross-react and bind more than one antigen.
Also, an antigen is a molecule that binds to Ag-specific receptors, but cannot necessarily induce an immune response in the body by itself. Antigens are usually proteins, peptides (amino acid chains) and polysaccharides (chains of monosaccharides/simple sugars) but lipids and nucleic acids become antigens only when combined with proteins and polysaccharides. In general, saccharides and lipids (as opposed to peptides) qualify as antigens but not as immunogens since they cannot elicit an immune response on their own. Furthermore, for a peptide to induce an immune response (activation of T-cells by antigen-presenting cells) it must be a large enough size, since peptides too small will also not elicit an immune response.
The antigen may originate from within the body (“self-antigen”) or from the external environment (“non-self”). The immune system is supposed to identify and attack “non-self” invaders from the outside world or modified/harmful substances present in the body and usually does not react to self-antigens under normal homeostatic conditions due to negative selection of T cells in the thymus.Vaccines are examples of antigens in an immunogenic form, which are intentionally administered to a recipient to induce the memory function of adaptive immune system toward the antigens of the pathogen invading that recipient.
-
Antibody
An antibody (Ab), also known as an immunoglobulin (Ig), is a large, Y-shaped protein produced mainly by plasma cells that is used by the immune system to neutralize pathogens such as pathogenic bacteria and viruses. The antibody recognizes a unique molecule of the pathogen, called an antigen, via the Fab’s variable region. Each tip of the “Y” of an antibody contains a paratope (analogous to a lock) that is specific for one particular epitope (similarly, analogous to a key) on an antigen, allowing these two structures to bind together with precision. Using this binding mechanism, an antibody can tag a microbe or an infected cell for attack by other parts of the immune system, or can neutralize its target directly (for example, by inhibiting a part of a microbe that is essential for its invasion and survival). Depending on the antigen, the binding may impede the biological process causing the disease or may activate macrophages to destroy the foreign substance. The ability of an antibody to communicate with the other components of the immune system is mediated via its Fc region (located at the base of the “Y”), which contains a conserved glycosylation site involved in these interactions. The production of antibodies is the main function of the humoral immune system.Antibodies are secreted by B cells of the adaptive immune system, mostly by differentiated B cells called plasma cells. Antibodies can occur in two physical forms, a soluble form that is secreted from the cell to be free in the blood plasma, and a membrane-bound form that is attached to the surface of a B cell and is referred to as the B-cell receptor (BCR). The BCR is found only on the surface of B cells and facilitates the activation of these cells and their subsequent differentiation into either antibody factories called plasma cells or memory B cells that will survive in the body and remember that same antigen so the B cells can respond faster upon future exposure. In most cases, interaction of the B cell with a T helper cell is necessary to produce full activation of the B cell and, therefore, antibody generation following antigen binding. Soluble antibodies are released into the blood and tissue fluids, as well as many secretions to continue to survey for invading microorganisms.
Antibodies are glycoproteins belonging to the immunoglobulin superfamily. They constitute most of the gamma globulin fraction of the blood proteins. They are typically made of basic structural units—each with two large heavy chains and two small light chains. There are several different types of antibody heavy chains that define the five different types of crystallisable fragments (Fc) that may be attached to the antigen-binding fragments. The five different types of Fc regions allow antibodies to be grouped into five isotypes. Each Fc region of a particular antibody isotype is able to bind to its specific Fc Receptor (except for IgD, which is essentially the BCR), thus allowing the antigen-antibody complex to mediate different roles depending on which FcR it binds. The ability of an antibody to bind to its corresponding FcR is further modulated by the structure of the glycan(s) present at conserved sites within its Fc region. The ability of antibodies to bind to FcRs helps to direct the appropriate immune response for each different type of foreign object they encounter. For example, IgE is responsible for an allergic response consisting of mast cell degranulation and histamine release. IgE’s Fab paratope binds to allergic antigen, for example house dust mite particles, while its Fc region binds to Fc receptor ε. The allergen-IgE-FcRε interaction mediates allergic signal transduction to induce conditions such as asthma.Though the general structure of all antibodies is very similar, a small region at the tip of the protein is extremely variable, allowing millions of antibodies with slightly different tip structures, or antigen-binding sites, to exist. This region is known as the hypervariable region. Each of these variants can bind to a different antigen. This enormous diversity of antibody paratopes on the antigen-binding fragments allows the immune system to recognize an equally wide variety of antigens. The large and diverse population of antibody paratope is generated by random recombination events of a set of gene segments that encode different antigen-binding sites (or paratopes), followed by random mutations in this area of the antibody gene, which create further diversity. This recombinational process that produces clonal antibody paratope diversity is called V(D)J or VJ recombination. Basically, the antibody paratope is polygenic, made up of three genes, V, D, and J. Each paratope locus is also polymorphic, such that during antibody production, one allele of V, one of D, and one of J is chosen. These gene segments are then joined together using random genetic recombination to produce the paratope. The regions where the genes are randomly recombined together is the hyper variable region used to recognise different antigens on a clonal basis.
Antibody genes also re-organize in a process called class switching that changes the one type of heavy chain Fc fragment to another, creating a different isotype of the antibody that retains the antigen-specific variable region. This allows a single antibody to be used by different types of Fc receptors, expressed on different parts of the immune system.
-
Antigen (noun)
A substance that induces an immune response, usually foreign.
-
Antibody (noun)
A protein produced by B-lymphocytes that binds to a specific antigen.
-
Antigen (noun)
a toxin or other foreign substance which induces an immune response in the body, especially the production of antibodies.
-
Antibody (noun)
a blood protein produced in response to and counteracting a specific antigen. Antibodies combine chemically with substances which the body recognizes as alien, such as bacteria, viruses, and foreign substances in the blood.
