Main Difference
The main difference between Amide and Amine is that the Amide is a group of chemical substances and Amine is a organic compound that is a derivative of ammonia.
-
Amide
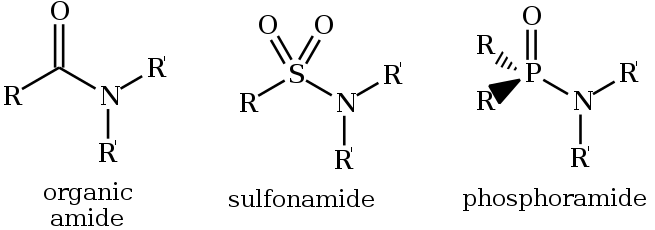
An amide ( or or ), also known as an acid amide, is a compound with the functional group RnE(O)xNR′2 (R and R′ refer to H or organic groups). Most common are carboxamides (organic amides) (n = 1, E = C, x = 1), but many other important types of amides are known, including phosphoramides (n = 2, E = P, x = 1 and many related formulas) and sulfonamides (E = S, x = 2). The term amide refers both to classes of compounds and to the functional group (RnE(O)xNR′2) within those compounds.
Amide can also refer to the conjugate base of ammonia (the anion H2N−) or of an organic amine (an anion R2N−). For discussion of these “anionic amides”, see Alkali metal amides.
Due to the dual use of the word ‘amide’, there is debate as to how to properly and unambiguously name the derived anions of amides in the first sense (i.e., deprotonated acylated amines), a few of which are commonly used as nonreactive counterions.The remainder of this article is about the carbonyl–nitrogen sense of amide.
-
Amine
In organic chemistry, amines (, UK also ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as chloramine (NClH2); see Category:Inorganic amines.The substituent -NH2 is called an amino group.Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure R–CO–NR′R″, are called amides and have different chemical properties from amines.
-
Amide (noun)
Any derivative of an oxoacid in which the hydroxyl group has been replaced with an amino or substituted amino group; especially such derivatives of a carboxylic acid, the carboxamides.
-
Amide (noun)
Any ionic derivative of ammonia in which a hydrogen atom has been replaced with a metal cation (R-NH– or R2N–)
-
Amine (noun)
A functional group formally derived from ammonia by replacing one, two or three hydrogen atoms with hydrocarbon or other radicals.
-
Amine (noun)
Any organic compound containing an amine functional group.
-
Amide (noun)
an organic compound containing the group —C(O)NH₂, derived from ammonia by replacement of a hydrogen atom by an acyl group.
-
Amide (noun)
a compound derived from ammonia by replacement of a hydrogen atom by a metal, containing the anion NH₂⁻
“sodium amide”
-
Amine (noun)
an organic compound derived from ammonia by replacement of one or more hydrogen atoms by organic groups.