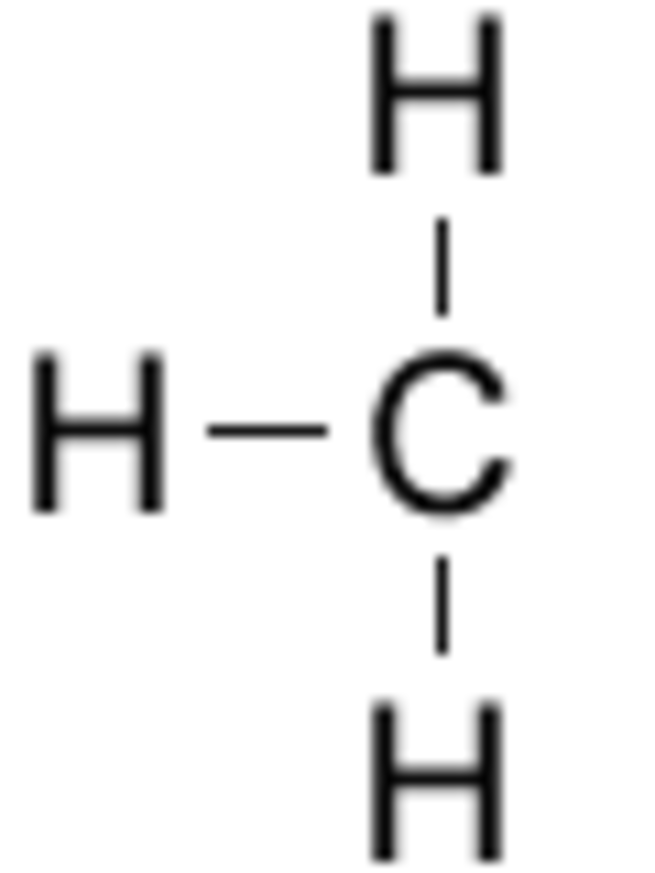
-
Alkyl
In organic chemistry, an alkyl substituent is an alkane missing one hydrogen.
The term alkyl is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula CnH2n+1. A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula CnH2n-1.
Typically an alkyl is a part of a larger molecule. In structural formula, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula CH3−.
-
Aryl
In the context of organic molecules, aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. “Aryl” is used for the sake of abbreviation or generalization, and “Ar” is used as a placeholder for the aryl group in chemical structure diagrams.
A simple aryl group is phenyl (with the chemical formula C6H5), a group derived from benzene. Examples of other aryl groups consist of:
The tolyl group, CH3C6H4, which is derived from toluene (methylbenzene)
The xylyl group, (CH3)2C6H3, which is derived from xylene (dimethylbenzene)
The naphthyl group, C10H8, which is derived from naphthaleneArylation is the process in which an aryl group is attached to a substituent. It is typically achieved by cross-coupling reactions.
-
Alkyl (noun)
Any of a series of univalent radicals of the general formula CnH2n+1 derived from aliphatic hydrocarbons.
-
Aryl (noun)
Any univalent organic radical derived from an aromatic hydrocarbon by removing a hydrogen atom.
