-
Nitrite
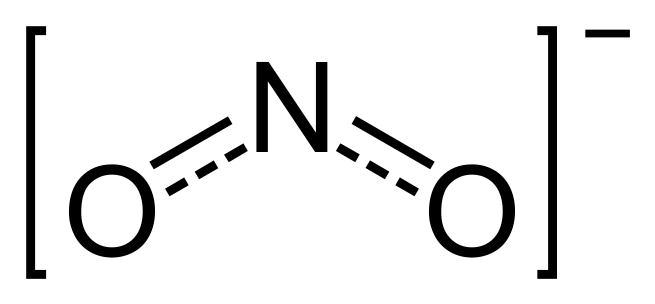
The nitrite ion, which has the chemical formula NO−2, is a symmetric anion with equal N–O bond lengths. Upon protonation, the unstable weak acid nitrous acid is produced. Nitrite can be oxidized or reduced, with the product somewhat dependent on the oxidizing/reducing agent and its strength. The nitrite ion is an ambidentate ligand, and is known to bond to metal centers in at least five different ways. Nitrite is also important in biochemistry as a source of the potent vasodilator nitric oxide. In organic chemistry the NO−2 group is present in nitrous acid esters and nitro compounds. Nitrite (mostly sodium nitrite) is also used in the food production industry for curing meat.Nitrate or nitrite (ingested) under conditions that result in endogenous nitrosation has been classified as “Probably carcinogenic to humans” (Group 2A) by International Agency for Research on Cancer (IARC), the specialized cancer agency of the World Health Organization (WHO) of the United Nations.
-
Nitrate
Nitrate is a polyatomic ion with the molecular formula NO−3 and a molecular mass of 62.0049 u. Organic compounds that contain the nitrate ester as a functional group (RONO2) are also called nitrates.
-
Nitrite (noun)
Any salt or ester of nitrous acid
-
Nitrite (noun)
The univalent radical -NO2, and the anion NO2–
-
Nitrate (noun)
Any salt or ester of nitric acid.
-
Nitrate (verb)
To treat, or react, with nitric acid or a nitrate
-
Nitrite (noun)
a salt or ester of nitrous acid, containing the anion NO₂⁻ or the group —NO₂.
-
Nitrate (noun)
a salt or ester of nitric acid, containing the anion NO₃⁻ or the group —NO₃
“preserved meat and vegetables contain nitrates”
“fish-fry populations are damaged by nitrate”
-
Nitrate (verb)
treat (a substance) with nitric acid, especially so as to introduce nitro groups
“a powerful nitrating agent”
“nitrated polycyclic aromatic hydrocarbons”
