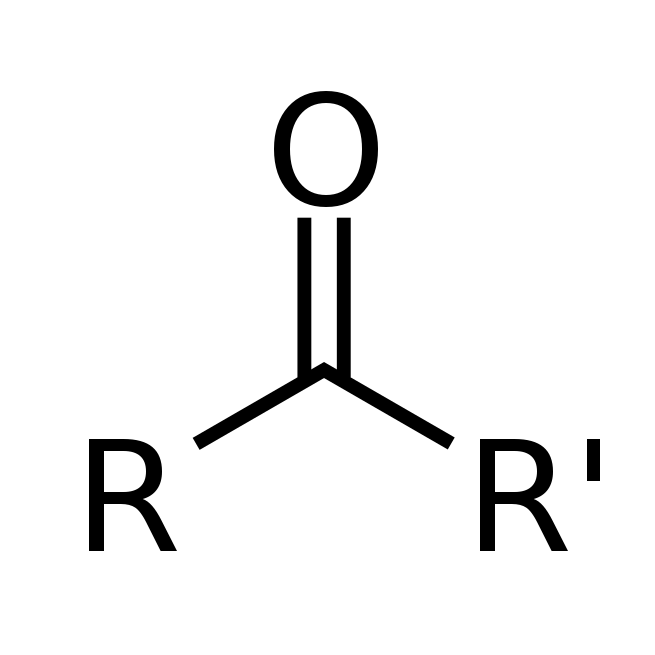
Main Difference
The main difference between Ketone and Aldehyde is that the Ketone is a class of organic compounds having structure RCOR´ and Aldehyde is a organic compound containing a functional group with the structure −CHO, consisting of a carbonyl center (a carbon double-bonded to oxygen) with the carbon atom also bonded to hydrogen and to an R group, which is any generic alkyl or side chain
-
Ketone
In chemistry, a ketone (alkanone) is an organic compound with the structure RC(=O)R’, where R and R’ can be a variety of carbon-containing substituents. Ketones and aldehydes are simple compounds that contain a carbonyl group (a carbon-oxygen double bond). They are considered “simple” because they do not have reactive groups like −OH or −Cl attached directly to the carbon atom in the carbonyl group, as in carboxylic acids containing −COOH. Many ketones are known and many are of great importance in industry and in biology. Examples include many sugars (ketoses) and the industrial solvent acetone, which is the smallest ketone.
-
Aldehyde
An aldehyde is an organic compound containing a functional group with the structure −CHO, consisting of a carbonyl center (a carbon double-bonded to oxygen) with the carbon atom also bonded to hydrogen and to an R group, which is any generic alkyl or side chain. The group—without R—is the aldehyde group, also known as the formyl group. Aldehydes are common in organic chemistry, and many fragrances are aldehydes.
-
Ketone (noun)
A homologous series of organic molecules whose functional group is an oxygen atom joined to a carbon atom—by a double bond—in a carbon-hydrogen based molecule.
-
Aldehyde (noun)
Any of a large class of reactive organic compounds (R·CHO) having a carbonyl functional group attached to one hydrocarbon radical and a hydrogen atom.
-
Aldehyde (noun)
an organic compound containing the group —CHO, formed by the oxidation of alcohols. Typical aldehydes include methanal (formaldehyde) and ethanal (acetaldehyde).
