-
Ketohexose
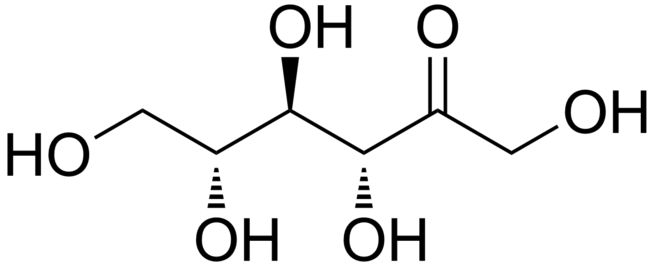
A ketohexose is a ketone-containing hexose (a six-carbon monosaccharide). The most common ketohexoses, each of which represents a pair of enantiomers (D- and L-isomers), include psicose, fructose, sorbose, and tagatose. Ketohexose is stable over a wide pH range, and with a primary pKa of 10.28, will only deprotonate at high pH, so is marginally less stable than aldohexose in solution. The basic formula for all ketohexoses is seen as
C
6
H
12
O
6
{displaystyle {ce {C6H12O6}}}
with a molecular weight of 180.156 g/mol. The different variations of this type of molecule come from the varying R and S chirality around the third, fourth and fifth carbon.Though all of these share similar structures, each have their own use and properties. Fructose in both L and D configurations is soluble in water, alcohol and ether. D-Sorbose is commonly used in the commercial synthesis of ascorbic acid. D-Tagatose is a rare natural ketohexose that is found in small quantities in food and has various health benefits.
-
Hexose
In bio-organic chemistry, a hexose is a monosaccharide with six carbon atoms, having the chemical formula C6H12O6. Hexoses are classified by functional group, with aldohexoses having an aldehyde at position 1, and ketohexoses having a ketone at position 2.
-
Ketohexose (noun)
Any hexose containing a ketone group.
-
Hexose (noun)
A sugar or saccharide containing six carbon atoms.
“Glucose is a common hexose”
-
Hexose (noun)
any of the class of simple sugars whose molecules contain six carbon atoms, such as glucose and fructose. They generally have the chemical formula C₆H₁₂O₆.