-
Hydroxide
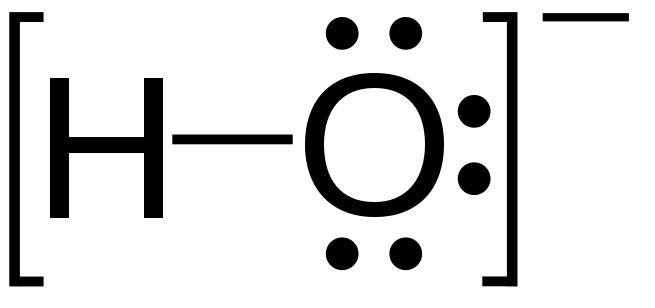
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It functions as a base, a ligand, a nucleophile, and a catalyst. The hydroxide ion forms salts, some of which dissociate in aqueous solution, liberating solvated hydroxide ions. Sodium hydroxide is a multi-million-ton per annum commodity chemical. A hydroxide attached to a strongly electropositive center may itself ionize, liberating a hydrogen cation (H+), making the parent compound an acid.
The corresponding electrically neutral compound HO• is the hydroxyl radical. The corresponding covalently-bound group –OH of atoms is the hydroxy group.
Hydroxide ion and hydroxy group are nucleophiles and can act as a catalysts in organic chemistry.
Many inorganic substances which bear the word “hydroxide” in their names are not ionic compounds of the hydroxide ion, but covalent compounds which contain hydroxy groups.
-
Hydroxide (noun)
An univalent anion (OH–) based on the hydroxyl functional group.
-
Hydroxide (noun)
Any substance containing such an anion.
-
Hydroxyl (noun)
A univalent radical or functional group (–OH) in organic chemistry; present in alcohols, phenols, carboxylic acids and certain other classes of compounds.
